Henderson-Hasselbalch Equation
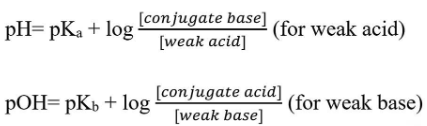
The Henderson-Hasselbalch equation is used to determine the pH of a buffer solution based on the amounts of weak acid and conjugate base or weak base and conjugate acid present. The equation is an approximation and only holds when the concentrations of acid and base at equilibrium have changed by less than five percent of their initial concentrations.
Weak acid and its conjugate base
Enter any three variables out of pKa, weak acid concentration, conjugate base concentration, and pH to calculate the fourth variable:
Weak base and its conjugate acid
Enter any three variables out of pKb, weak base concentration, conjugate acid concentration, and pOH to calculate the fourth variable: